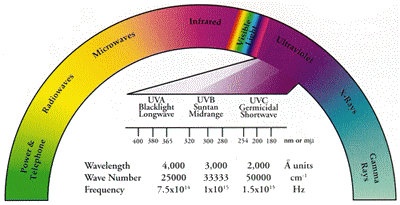
 An important thing to know is that ultraviolet (UV) is not
a single entity, but a wide band of wavelengths. The chief natural source of
UV is the sun, most of which is in the region between 300nm - 400nm.
Artificial sources of UV include incandescent, gas discharge, low pressure mercury,
medium pressure mercury metal halide, electrodeless and xenon lamps.
An important thing to know is that ultraviolet (UV) is not
a single entity, but a wide band of wavelengths. The chief natural source of
UV is the sun, most of which is in the region between 300nm - 400nm.
Artificial sources of UV include incandescent, gas discharge, low pressure mercury,
medium pressure mercury metal halide, electrodeless and xenon lamps.
UV radiation is electromagnetic radiation in the part of the spectrum between x-rays and visible light. It differs from visible light only in that the UV wavelengths are too short to be seen by the human eye. The boundary between visible light and UV is a wavelength of 400nm (4000 Angstroms). Medical literature divides UV into three ranges: UVA (315 - 400nm) where the filtered (BLB) is used for fluorescence blacklight effects while unfiltered UVA is used in UV curing and photochemical reactions. UVB (280 - 315nm) is used in scientific applications such as genetic visualization. UVC (200 - 280nm) unfiltered UV is used in germicidal crosslinking applications and when properly filtered, for mineral fluorescence. This is a small sampling of applications. Contact WaterTec for assistance with your application. An interesting characteristic of UV radiation occurs when it falls upon certain substances known as phosphors, where it causes the phosphors to emit specific radiation. This phenomenon is known as fluorescence. Everyday fluorescent lighting is basically a UV lamp constructed of a type of glass bulb that blocks UV rays. The inside of the bulb is coated with a thin layer of fluorescent material that receives UV generated by the lamp and in return emits a visible light. One effect of ultraviolet energy upon certain substances is a phenomenon that takes place at the atomic level. High frequency UV photons collide with atoms and part of the photon energy is transferred to the atoms by boosting electrons to the high energy states. Upon de-excitation, as electrons fall back to lower energy states, energy is released as photons of light. Since only a portion of the incoming photon energy was transferred to an electron, these emitted photons have less energy than the incoming UV photons so their wavelengths are longer than the excitation photons. This process is called fluorescence. In some materials, the fluorescence lingers and disappears slowing after the UV source is removed. Here, the electron returns slowly to its original state, and this delayed fluorescence is call phosphorescence. |
 [ UV
| RO
| English Page
| WaterTec Home ]
[ UV
| RO
| English Page
| WaterTec Home ]

